The Alzheimer’s disease research field is heavily affected by the COVID-19 pandemic, with many activities being delayed. Restrictions have been put in place and the consequences of the pandemic have been multiple. Due to the global impact of COVID-19, the AMYPAD members had temporarily paused new patient enrollment in its two clinical studies. In addition, as the COVID-19 has spread around the globe, the pandemic has created a complex set of clinical trials recruitment challenges.
AMYPAD Diagnostic and Patient Management Study (DPMS):
The COVID-19 pandemic has impacted recruitment of new participants (9 participants in Q2 2020 vs 118 in Q1 2020). The recruitment period between January and February 2020 was very productive. However, from March the AMYPAD team has observed a drastic decrease in the recruitment of patients in all eight active sites due to the COVID-19 pandemic. Recruitment stopped in all sites from the second week of March to the end of April. Starting from May, recruitment has slowly restarted in some countries.
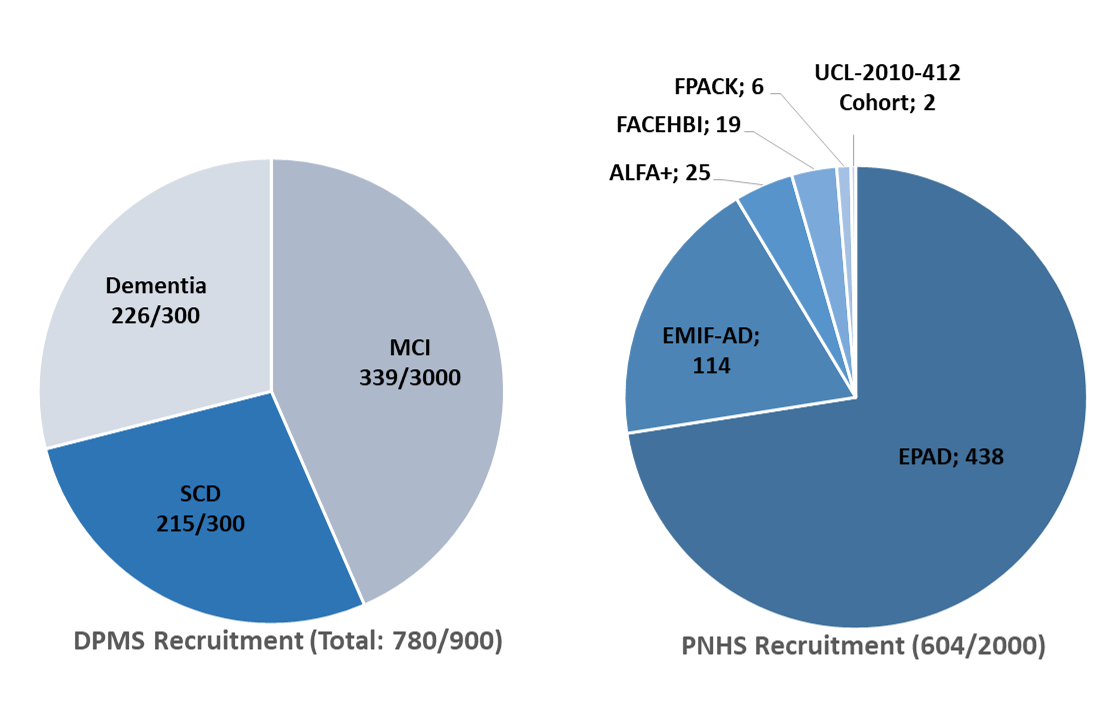
As of August 24th, 2020, 780 patients have been included and 616 scans performed (including 26 repeated scans). At the moment, the DPMS study includes 215 Subjective Cognitive Decline (SCD), 339 Mild Cognitive Impairment (MCD) and 226 dementia patients. The recruitment will be able to resume in all eight sites once the situation has stabilised, with an expected delay to reach the 900 patients. Before the COVID-19 pandemic, the DPMS study was on track with the number of recruiting sites and enrolled participants.
During the past months, the sites were able to focus on the data quality check and the team has continued to work on data cleaning to allow moving forward with the analysis. The analyses of the AMYPAD DPMS participants’ baseline features is ongoing and the preliminary results were presented last month at the virtual AAIC conference. You can download the poster here.
AMYPAD Prognostic and Natural History Study (PNHS):
Recruitment activity within the AMYPAD PNHS had also stopped during the COVID-19 crisis. This was due to the closure of sites and cessation of research visits. Consequently, the past months have seen slow progress within the PNHS in terms of recruitment. By June 2020 some AMYPAD PNHS sites had slowly started to resume recruitment, always taking into account local and national measures concerning safety and distancing. Of the 17 active sites in the PNHS, 12 sites have been able to resume recruitment, although at reduced capacity. It was expected that all sites could restart their activities in September; however, the current developments with COVID-19 could cause another period of site closures and slow activity.
Despite the difficulties, the PNHS has now reached 600 subjects consented, coming from six different Parent Cohorts. As of August 24th 2020, 903 participants have been informed about the study, 604 consented, and 448 already underwent their amyloid PET scan.
Over the past months, sites were able to focus on other study related activities such as any backlog of data entry, quantification and preparation of documents needed for the global amendment to Ethics Committees and Regulatory Authorities in order to allow for the continued recruitment and follow-up of former EPAD LCS participants. The first quantitative results of the AMYPAD PNHS were presented at the virtual AAIC conference. You can download the poster here.