The members of the Amyloid imaging to prevent Alzheimer’s disease (AMYPAD) consortium have announced the publication of a perspective review1 on the interpretability and clinical application of the Centiloid (CL) scale, a robust method for measuring amyloid plaques in the brain, a key hallmark of Alzheimer’s disease (AD). Published in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, this work includes the context-of-use recommendations and aims to provide guidelines for the implementation of the CL scale in clinical practice, ensuring its reliability and accuracy for diagnosing and monitoring AD across different settings.
Dr. Lyduine Collij, Researcher at Amsterdam UMC and Lund University, commented that “we have summarised the available literature and AMYPAD’s Centiloid Working group endeavors to propose context-of use recommendations regarding the clinical use of Centiloid to prepare the European field for the arrival of disease-modifying therapies, which will require highly accurate diagnosis to avoid unnecessary burden for patients and their families and costs for our healthcare system.”
“In addition to the four Centiloid use scenarios we described, we set out to identify the remaining knowledge gaps and gathered first evidence of longitudinal investigations using the Centiloid scale, which is important as the field is moving towards early intervention.2” added Dr. Ariane Bollack, scientific advisor at GE HealthCare and honorary researcher at University College London.
In recent years, AMYPAD researchers have played a pivotal role in validating the CL scale as a robust and reliable method for quantifying amyloid deposition in the brain, independent of the radiotracer used in PET imaging. The CL scale provides a unified and standardised framework for comparing data across different PET imaging tracers and quantification pipelines, enabling a more accurate assessment of amyloid burden. This recently published AMYPAD work, conducted across multiple clinical sites, demonstrated improvement in diagnostic accuracy, particularly when assessing equivocal cases3.
Dr. Mahnaz Shekari, researcher at the Neuroimaging Research Group at the BBRC center, explains that “we have demonstrated the precision of the Centiloid metric in memory clinic patients, establishing it as a reliable biomarker for diagnosing and tracking Alzheimer’s disease. Furthermore, we offer a comprehensive, easy-to-follow guideline for using the Centiloid metric in amyloid PET quantification, making it accessible to both clinicians and researchers.”
These findings have been consolidated into an application for a Biomarker Qualification Opinion (BQO) to the European Medicines Agency (EMA). The EMA’s Committee for Medicinal Products for Human Use (CHMP) has recognised the Centiloid unit for measuring brain amyloid levels as a validated measure of global amyloid load in the brain, if properly used with quality control procedures4.
“The qualification opinion is a regulatory endorsement of this methodology. It lends credibility for it to be used as an outcome measure in clinical trials, and it’s a stepping stone to possible future use in routine clinical practice.”, said Gill Farrar, medical director at GE HealthCare, who was the project lead of AMYPAD on the industry side.
The emerging era of anti-amyloid therapies relies heavily on amyloid-PET imaging for patient selection, evaluation of target engagement and assessment of drug effectiveness. For these therapies, the Centiloid scale offers a robust, tracer-independent and validated metric that accurately reflects the degree of amyloid pathology in the brain, making it an ideal tool for use in clinical settings. According to Frederik Barkhof, professor of Neuroradiology at VU University Medical Centre, Amsterdam and University College London, who was the academic lead of the AMYPAD project “There are treatments being developed for Alzheimer’s disease that remove amyloid plaques from the brain. When this works, there is less amount of radioactive tracer visible on the scan. People can return to an almost normal scan, so – using the Centiloid scale – the effect of the treatment can now be measured.”
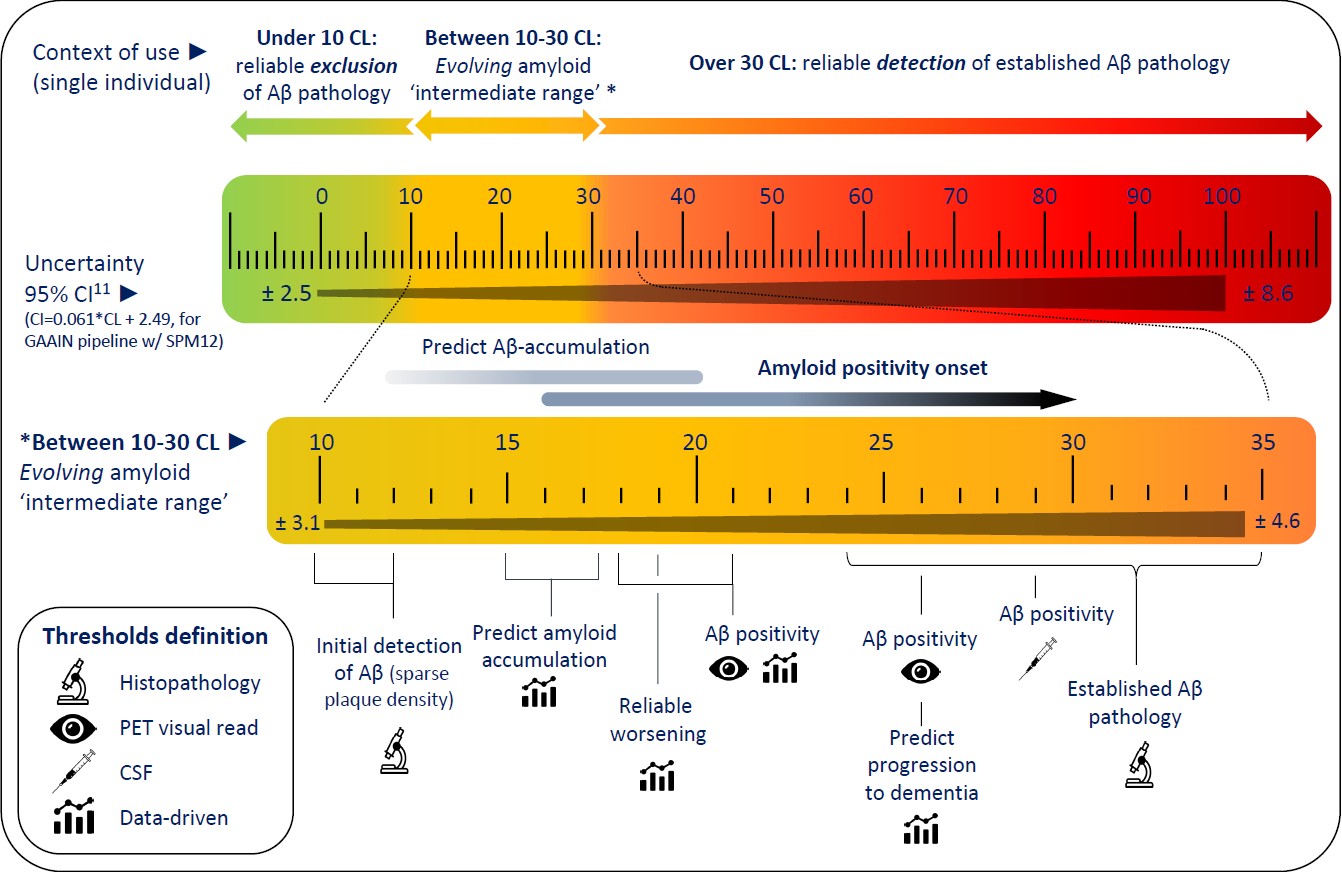
Figure: Overview of Centiloid scale interpretation
Illustrative figure summarising the main studies regarding CL based cut points to reliability exclude (<10 CL) and detect (>30 CL) Aβ-pathology at the individual level. In addition, the 95% confidence interval across the scale are provided and detailed cut-offs in the ‘intermediate range’ are highlighted. (CL: Centiloid; CI: Confidence interval; CSF: cerebrospinal fluid)
Bibliographic reference
- Lyduine E. Collij, Ariane Bollack et al. “Centiloid recommendations for clinical context-of-use from the AMYPAD consortium”. Alzheimer’s & Dementia.2024;1-12. https://doi.org/10.1002/alz.14336
- Bollack, Ariane, et al. “Investigating reliable amyloid accumulation in Centiloids: Results from the AMYPAD Prognostic and Natural History Study.” Alzheimer’s & Dementia.2024;20:3429–3441.
https://doi.org/10.1002/alz.13761 - Mahnaz Shekari et al. “Stress testing the Centiloid: Precision and variability of PET quantification of amyloid pathology.” Alzheimer’s & Dementia.2024;20:5102–5113. https://doi.org/10.1002/alz.13883
- EMA – Qualification opinion for Centiloid measure of Amyloid PET to quantify brain amyloid deposition (EMADOC-1700519818-1200791) https://www.ema.europa.eu/en/documents/other/qualification-opinion-centiloid-measure-amyloid-pet-quantify-brain-amyloid-deposition_en.pdf
You can download the press release here.