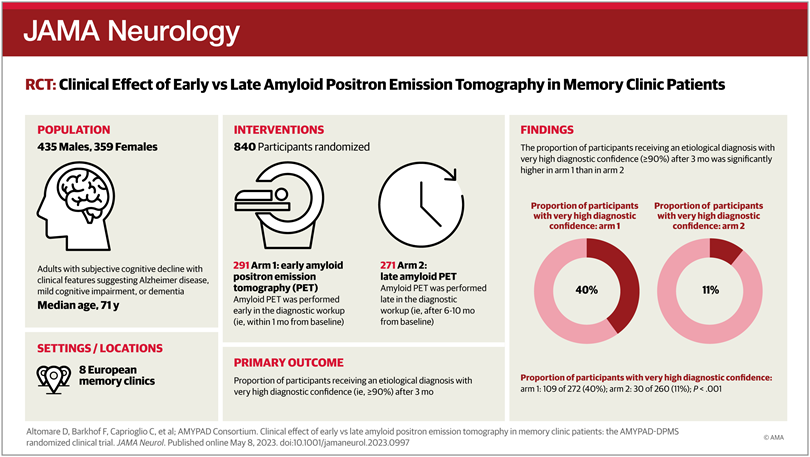
The AMYPAD Diagnostic and Patient Management Study (DPMS) was designed as a prospective, multicenter, randomised clinical trial assessing the clinical effect of amyloid PET in memory clinic patients. It aims to fill the current evidence gap by providing strong evidence on the clinical utility and cost-effectiveness of amyloid PET. A total of 840 patients from 8 European memory clinics have participated in this study.
“This is a prospective, multicenter, and randomized controlled study, and it is the largest in Europe to evaluate the clinical impact of this diagnostic tool in memory clinic participants,” explains Daniele Altomare, senior postdoctoral researcher and study coordinator at the Laboratory of Neuroimaging of Aging at the University of Geneva.
In a new paper published in JAMA Neurology, researchers investigated whether participants allocated to undergo amyloid PET early in their diagnostic workup received an etiological diagnosis with very high diagnostic confidence after 3 months more frequently than those who had not undergone amyloid PET yet. Moreover, they also assessed whether early amyloid PET is associated with more frequent changes in diagnosis, diagnostic confidence, and treatment plan. Finally, they examined the real-world use and clinical effect of unrestricted amyloid PET imaging in a free-choice group.
“Despite the increasing use of this test in clinical practice, real-world evidence on its clinical utility and cost-benefit ratio is still limited. In fact, although several studies have been published, this is the first randomized controlled clinical trial carried out to confirm the clinical usefulness of amyloid PET,” says Juan Domingo Gispert, head of the Neuroimaging Research Group of the BBRC, which led the center’s participation in AMYPAD.
The study showed that access to this diagnostic test resulted in an etiological diagnosis with very high certainty in 40% of patients, within three months of initial clinic visit. This corresponds to a percentage of 3.5 times higher than those who had not undergone amyloid PET. Moreover, amyloid PET changed the initial diagnosis in 44% of cases, compared to only 11% in the group without amyloid PET.
“This new clinical trial provides strong evidence supporting the early implementation of this test, as its use is associated with accurate diagnoses,” says Giovanni B. Frisoni, director of the Center for Memory at the University Hospital of Geneva and principal investigator of the study. “A safe and reliable diagnosis is essential for the efficacy of disease-modifying therapies, especially anti-amyloid drugs, whose effectiveness could decrease with the progression of the disease”, he assures.
Congratulations to all authors!
You can read the paper here.
You can read the press release prepared by our partner BBRC here.